Lithium production is facing a transformation. Mining, automotive and chemical companies around the world are in a race to improve the efficiency of production processes and reduce the environmental impact of the dominant methods of lithium extraction, in order to meet the exponential growth in demand associated with the scale-up of electric vehicles.
“Traditional brine processes have a high environmental impact, due to high levels of water evaporation,” says researcher Michelle Lee Yin of the Pontifical Catholic University of Chile. “There are different alternative technologies with the potential to replace and/or support the current production method, with the aim of achieving a more sustainable industry over time.”
Some of these alternative production methods are applied in a way that complements the traditional approaches, though most are still in the laboratory phase. In all cases, the scale of production required at the industrial level presents major challenges in terms of consumption, water recovery, waste generation and electricity use.
Evaporating a ‘complex soup’
In the high altitude salar, the salt flats, of Argentina and Chile, the predominant method of lithium extraction is by evaporation and the addition of lime and sodium. It consists of pumping brine from the depths of the salt flats and then concentrating it in large pools for 12 to 18 months. The brine is a “complex soup” in which there is a great variety of salts, and lithium is a minority. Each of these salts has a different solubility and the final element remaining after more than a year is lithium.
The resulting liquid, rich in lithium chloride, is then piped to a chemical plant. There, solvents are applied, and a filtering process is carried out to derive lithium carbonate, a solid. Subsequently, the low-purity lithium carbonate is washed and dried, to reach battery-grade lithium, which implies a purity of more than 99%.
In Chile’s Salar de Atacama, the two companies extracting lithium, domestic firm SQM and US-based Albemarle, use the traditional evaporation method. Similarly, in Argentina, Australia’s Orocobre (associated with Toyota Tsusho of Japan) also extracts lithium from the Salar de Olaroz in Jujuy province using the conventional evaporation method. From this process it produces lithium carbonate, then adds value by transforming it into hydrated lithium hydroxide or battery-grade lithium carbonate in Japan.

The Minera Exar project, in Argentina’s Cauchari-Olaroz salt flat is about to begin production, formed by Canada-headquartered Lithium Americas, Chinese mining giant Ganfeng and JEMSE, the Jujuy state-owned company. The project will use the traditional evaporation method to then achieve battery-grade lithium carbonate in its chemical plant.
Meanwhile, the Korean company Posco will have 400 hectares of evaporation pools on Argentina’s Salar del Hombre Muerto, in Catamarca province, to obtain lithium phosphate. It will then transport the concentrate to a plant in neighbouring Salta province to produce lithium hydroxide for the first time in the country.
Evaporation in a desert
As lithium has a very low concentration in brine, a massive volume is required to achieve high production values, such as those required for electric vehicles.
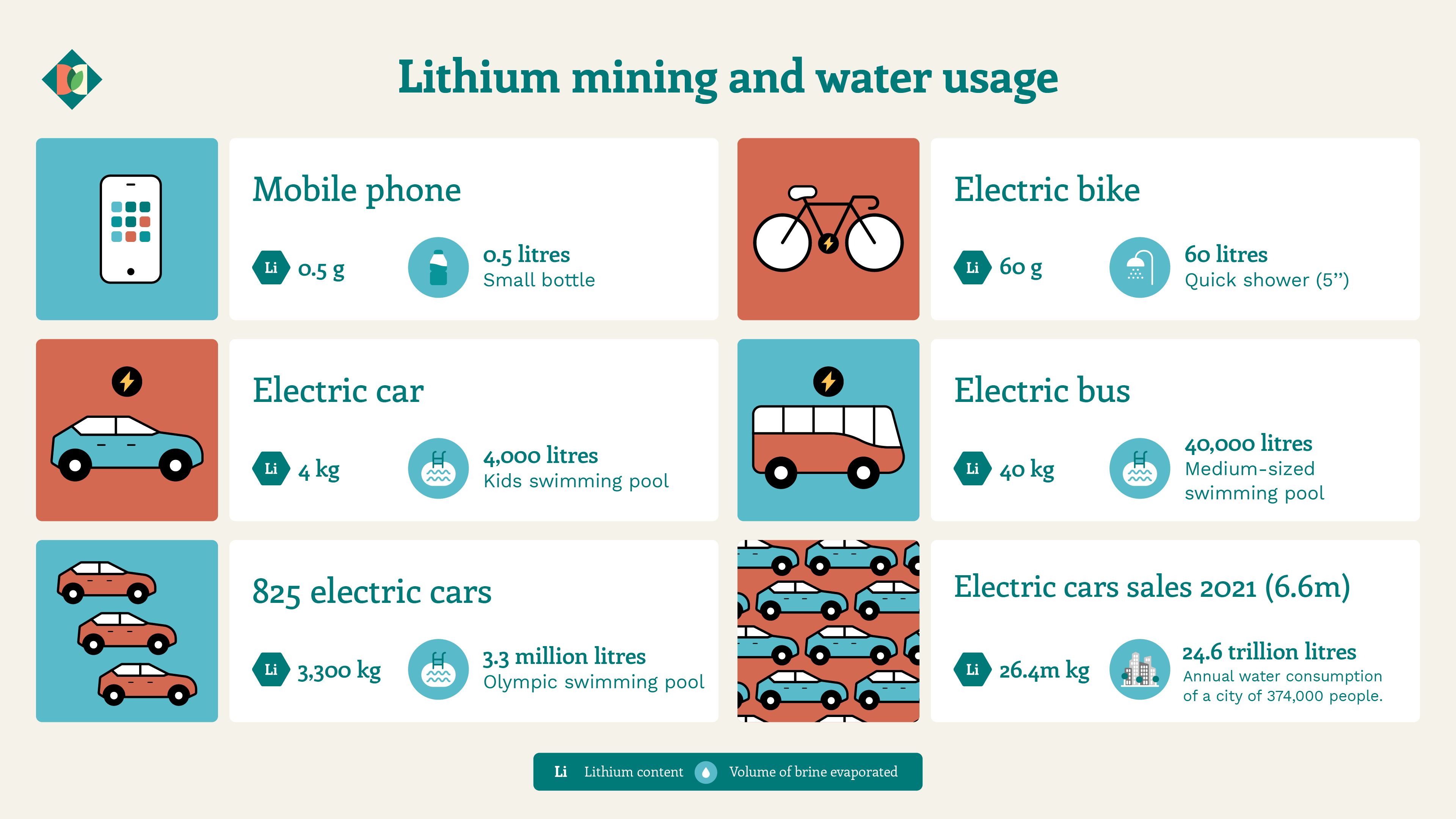
Ernesto Calvo, an Argentine scientist and expert in the sector, explains that at typical concentrations of 500 to 1,000 mg of lithium per litre of brine, around 200,000 litres of brine must be evaporated to extract one tonne of lithium carbonate equivalent. “This method worked for the volumes demanded in the mobile phone business, but the scale demanded by electric cars makes it unsustainable. We are giving ourselves the luxury of evaporating water in the middle of a desert,” he warns.
Meanwhile, Lee Yin calculates that if around 150,000 tonnes of lithium carbonate are currently produced annually in Chile this means that 25 million cubic metres of water are evaporated every year – an amount that will only increase as lithium demand increases. “By 2025, the amount of water evaporated in the lithium industry will almost reach the drinking water consumption of Chile’s Antofagasta region,” she says.
This method worked for the volumes demanded for mobile phones, but the scale demanded by electric cars makes it unsustainable. We are giving ourselves the luxury of evaporating water in the middle of a desert
It should be clarified that this is not water that is used directly for human or animal consumption, but rather the water contained in the brine. However, studies indicate that over-extraction of this liquid can generate local climatic changes and modify the natural evaporation rate of the system, as the interactions between the different hydrogeological systems must be considered. For example, brine extraction may cause freshwater inflow to replace the volume extracted, which would affect water sources for human or agricultural use.
Pía Marchegiani, director of environmental policy at the Fundación Ambiente y Recursos Naturales (FARN), an Argentine NGO, warns that “the debate on the environmental impact of these operations on fragile ecosystems can be completed when there is sufficient and independent environmental information on the functioning of the complex hydrogeological system in which the projects are located.”
Other lithium extraction techniques
The primary impetus for companies to adopt alternative extraction technologies to the evaporation method is to improve the efficiency of the production process, which impacts the balance between the initial investment and the amount of lithium sold. Part of the lithium contained in the brine is also lost during the evaporation process.
Some estimates suggest that only 30-40% of lithium at mining sites is extracted, due to inefficiencies in the process
According to Albemarle, which operates in Chile, the loss of lithium from its operations is as high as 45%, while other estimates calculate that the industry operates with efficiency levels in the order of 30–40%. In addition, extraction is a very long production process, taking between 12 and 18 months, which also has impacts on the cost.
Unlike in Chile, the first lithium exploitation project in Argentina, carried out by the US company FMC, now called Livent, in the Salar del Hombre Muerto, uses a direct extraction process based on absorption columns composed of gibbsite, a mineral form of aluminium hydroxide, which allows lithium to be “filtered” in a selective way.
This method consists of brine pumped from the salar being filtered through a 25-tonne column that traps the lithium and allows the other compounds to flow through. The columns are then flushed with water to release the trapped lithium. However, in order to increase the scale of production, since 2012, Livent has been pre-concentrating lithium by evaporating brine in pools.
Within the range of new lithium projects that will soon begin production in the country, the French company Eramet will also use an absorption technique, similar to the one used by Livent. According to Daniel Chávez, CEO of subsidiary Eramine Sudamérica, “in the short term, perhaps a handful of years, all lithium extraction will be carried out using new methods, mainly because a lot of lithium is lost during the evaporation process of the traditional method.”
Australian company Rio Tinto recently made an US$825 million investment to start developing the Rincón brine lithium project in Salta, and will also use a direct extraction method.
The race for lithium
With a horizon of high prices and growing global demand, alongside environmental and efficiency difficulties of traditional extraction methods, the industry is in a race of research, trial and error around new production methods.
Direct extraction methods, including the absorption method used by Livent and Eramine, are based on a more selective chemical strategy that seeks to separate lithium from other compounds more quickly, through a process that takes hours, compared to the 12 to 18 months required for evaporation. In addition, they have an efficiency of 70-90%, extracting much more lithium than is available from the evaporation method.
However, each of the new methods has its own complexities to deal with, from the heavy use of freshwater in the plant to separate the lithium, to the generation of waste due to the use of solvents, and the intensive use of electricity.

“The new methods are in pilot testing, except for the absorption columns, which are more advanced. They are all more efficient and much faster. They are not very complex chemical processes, but they are very large volumes, at an altitude of 4,000 metres, with a huge temperature difference between day and night,” explains Calvo, who led the development of a method that passed laboratory tests. This process uses electric current as a way to “select” the lithium from the brine. It uses no water and generates no waste, and foresees the use of energy through solar panels.
It is not only mining companies that are behind the new production methods. General Motors, Tesla, BMW, the oil company Schlumberger, Panasonic and Renault, among other big players, are also in the race.
Argentine researchers Andrés López, Martín Obaya, Paulo Pascuini and Adrián Ramos state that “among the most advanced techniques” is one developed by the Israeli firm Tenova Advanced Technologies, based on the use of a solvent that, according to the company, achieves a lithium chloride solution with a purity of more than 99.9% in just one day. The process involves re-injection of the lithium-free brine back into the salt, a complex process not yet used on an industrial scale.
Another method under investigation involves the use of a membrane composed of certain materials that are attractive to lithium, which by means of water vapour allows the separation of salts.
FARN’s Marchegiani argues that the possibility of advancing in lithium exploitation with techniques that use less water initially seems like good news, in the context of water scarcity in the Atacama Plateau, where much of Chile and Argentina’s lithium is located. However, she calls for a comprehensive evaluation: “For now, many of them only work as laboratory tests or pilots; we will have to see if they are possible on an industrial scale.”








